Crystallization
Some metallurgical processes involve phase transition.
The typical example of phase transition is crystallization.
Crystallization is transformation of liquid phase to solid crystalline phase.
There are two general stages of phase transformation (crystallization) process – nucleation and growth:
Nucleation
Nucleation is a process of formation of stable crystallization centers of a new phase.
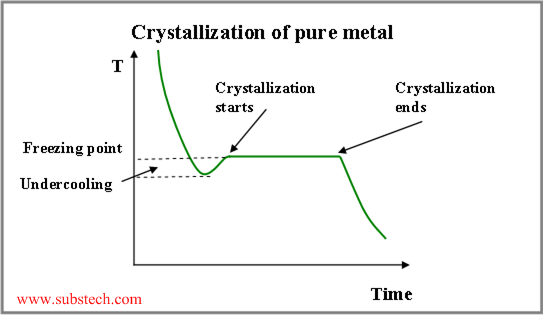
Nucleation may occur by either homogeneous or heterogeneous mechanism, depending on the value of undercooling of the liquid phase (cooling below the equilibrium freezing point).
Presence of foreign particles or other foreign substance in the liquid alloy (walls of the casting mold) allows to initiate crystallization at minor value of undercooling (few degrees below the freezing point). This is heterogeneous nucleation.
If there is no solid substance present, undercooling of a hundred degrees is required in order to form stable nuclei or “seeds” crystals, providing following crystal growth (homogeneous nucleation)
Undercooling value determines quantity of nuclei, forming in the crystallizing alloy. When a liquid comes into a contact with cold and massive mold wall (chill zone), it cools fast below the freezing point, resulting in formation of a large quantity of stable nuclei crystals.
In order to promote the nucleation process, surface-active additives are used. They decrease interfacial energy of the nuclei crystals, causing formation of many more new stable nuclei.
to top
to top
Crystal growth
Number of stable nuclei per unit volume of crystallizing alloy determines the grain size.
When a large number of stable nuclei are present in chill zone of mold, fine equiaxed grains form. Latent crystallization heat, liberating from the crystallizing metal, decreases the undercooling of the melt and depresses the fast grains growth.
At this stage some of small grains, having favorable growth axis, start to grow in the direction opposite to the direction of heat flow. As a result columnar crystals (columnar grains) form.
Contrary to the pure metals, in alloys different type of undercooling takes place. It is called constitutional undercooling.
to top
to top
Constitutional undercooling
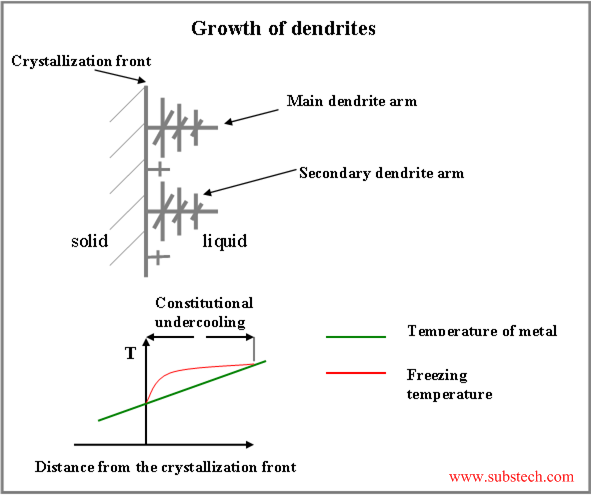
Since solubility of an alloying element in solid is lower, than in liquid at the same temperature, this element (solute) is rejected by the solidifying metal to the liquid phase, enriching the region of liquid adjacent to the crystallization front.
For the most of the alloys: the higher the concentration of alloying element in the alloy, the lower its liquidus temperature (temperature at which crystallization of the alloy starts).
Thus crystallization temperature of the liquid, adjacent to the crystallization front, rises with increasing the distance from the front surface. Therefore there is a layer of the liquid, where its temperature is lower, than its crystallization temperature. This is the region of constitutional undercooling (see the figure below).
to top
to top
Dendrites
If a protruding finger forms on the solidifying surface, its tip may reach the region of constitutional undercooling . In this case the protuberance starts accelerated growth, forming the main dendrite arms. Under certain conditions the same process may occur on the surface of the main dendrite arms, causing branching off the secondary arms and then arms of higher orders.
Process of solidification is considered in the article Solidification.
No comments:
Post a Comment